Nikola Tesla Articles
Cold Light
Cold light is one of the wonders of the modern age. Light without heat seems like a contradiction, nevertheless it is a scientific fact. Since the discovery of the electrical nature of light, the possibility of transforming electricity directly into light, without the production of heat, has been a tempting goal. For centuries, scientists dreamed of solving the mystery of the firefly and the Aurora Borealis, but dreamed in vain. They saw light without heat produced in Nature, but could not produce it themselves.
Today the discovery that light may be produced without heat, by means of electrical ionization, seems to be the key to the situation, and we can only wonder where it may lead us. At the present time over 96 per cent of all the energy used in the fifty million incandescent lamps in the United States, instead of being turned into light, is wasted in the form of heat! Moreover the astonishing production of light without heat by ionization has given us a new view of the relation between light and electricity and the great problem of the origin of light.
It used to be thought that light could only be produced by incandescence, that is, thru heating of a solid body to white heat. Strange as it may seem, the candle and the kerosene lamp are really incandescent lights. The light they give off is due to white-hot particles of carbon liberated in the combustion process. Even the light from an arc lamp is mainly due to the crater in the positive carbon being heated to incandescence.
The fact that heat is not the only factor which effects the production of light can be easily seen in two ways. First, salts of sodium, potassium and lithium may be thrown in the same flame at exactly the same temperature, but the first gives off a bright yellow light, the second a violet, and the third a deep red. Second, light is produced by an electric spark in a partial vacuum, such as a Geissler tube, with little or no heat at all. In both of these cases the light must be produced by the breaking up of the molecules, of the solid or gas, into electrically charged particles, or ions, in a manner to be explained later.
The idea of using cold light for illuminating purposes seems to have first occurred to Nikola Tesla. Very early in his career he suggested that the effects produced in Geissler tubes might be utilized in that way. Not until recent years, however, did his suggestion bear fruit in the shape of the now famous Moore vacuum tube light, invented by McFarland Moore, which produces a fine soft light from a glass tube thirty to forty feet long, filled with carbon dioxid. The heat which this light produces is almost negligible.
Cold light is a term used to indicate luminous radiation without heat radiation, and that means maximum efficiency, i.e., a colder and a colder source. The old fashioned kerosene lamp wastes 36.4 calories of heat per second for every candle- power of light produced. The gas mantle wastes 11, and the tungsten lamp but from 1, to 2, calories.
Considering electric lights only, the heat loss in tungsten lamps has been reduced by the introduction of gases such as nitrogen into the bulb, but arc lights have made a greater development. The invention of the enclosed arc reduced the heat loss without reducing the illumination. Then the invention of the flaming arc increased the illumination without increasing the temperature of the arc. The latter is the most interesting development because part of the light from the flaming arc is cold light, from the chemicals in the flame which are ionized by the electric current. The cold light from these ions plus the light from the incandescent carbons makes the flaming arc nearly three hundred per cent more efficient than the ordinary incandescent lamp.
The first flaming arc appeared in 1900, known as the Bremer arc, and using carbons impregnated with several chemical salts. Later Steinmetz and Whitney used the rare metals titanium and chromium in their arcs and produced a still more efficient light. About the same time the mercury vapor light was invented, which gave a brilliant bluish white light from the mercury ions in the arc. Later still is the discovery by two scientists, Franck and Hertz, that an electric current of only 4 volts, when past thru cold iodin vapor, produces a faint light of definite color. When the voltage is raised to twelve, another color is obtained. This is true cold light, produced directly from the molecules as they are ionized by the current. How this is possible, and how light and heat may be produced independently of each other, will now be shown.
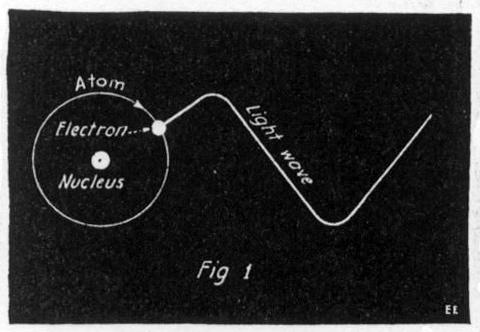
What the actual source of a light wave is has been one of the mysteries of science since the beginning of history. The discovery by Maxwell, Hertz and others that light is an electro-magnetic vibration, caused scientists to look for the electric charges which produce these waves. When the electron was discovered it was thought that the solution had been found, but there was still one great drawback. Scientists said that as the electron revolved about its axis in the molecule it produced light waves just like a paddle wheel in a body of water. But as all molecules of all substances contain electrons which are constantly in motion, then all substances should give off light all the time. This was ridiculous, as everything in the world and the whole universe would be of a dazzling brightness like the sun.
The discoveries of today are changing this idea completely. One of the world's greatest physicists, Planck, says electrons cannot give off light waves continuously, but emit definite quantities at intervals. This theory he calls the quanta theory, and another physicist, Bohr, says the time the light is given off is when the motion of the electron is suddenly changed from its normal continuous motion, by its jumping from one atom to another, or from one orbit to another about the same atom. This is a very simple theory, but it is often the simplest theory which best explains the facts. When trillions of trillions of electrons are doing this at the same time, the light is to all purposes continuous. These theories have received added confirmation from work done recently by Prof. Millikan of Chicago University, who gained fame by his original measurements of the electron itself.
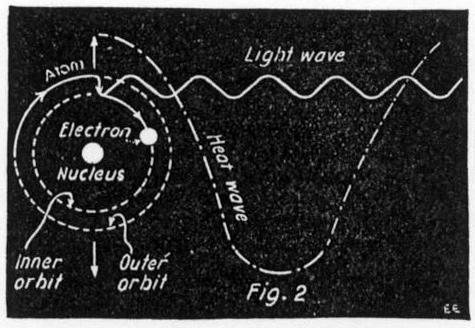
The principle of the new theory can be easily explained by an illustration. If a ball is whirled in a circle on the end of a string, the pull on the string is steady and continuous as long as the ball whirls with the same speed. However, if the ball is suddenly stopt or hit by something which makes it change its course, a jerk or a wave will be felt in the string. Now an electron does the same thing, only the jerk or wave is sent out in all directions by the electro-magnetic field about the electron. This jerk or wave is the little quantity which Planck calls a "quanta". The familiar way in which an electron is pictured as continuously producing light waves, by its rotation, is shown in Fig. 1, but this is wrong according to the above theory. What really must happen is shown in Fig. 2, where the two circles represent different orbits and the full line represents the path of the electron jumping from one orbit to another and at the same time emitting a quantity of radiation or light. The way in which electricity or some other agency causes this disturbance of the electron is not fully understood.
The most remarkable thing about it all is that heat and light waves may be produced independently. A heat wave is produced by the vibration of the whole molecule or atom as shown by the big arrows and dotted wave line in Fig. 2. And under proper conditions the small light wave may be given off by the electron without affecting the whole molecule in the least. That is cold light.
An interesting experiment can be performed with a vacuum tube, which illustrates completely the principle of light production by ionization, and at the same time explains the principle of the Moore light. Few people, other than scientists, realize what a peculiar thing the production of light in a vacuum or Geissler tube is. The light is evidently not the product of heat, but depends on the fact that the electric spark breaks the gas molecules up into charged particles or ions. And this phenomena is most noticeable when a certain degree of exhaustion is reached in the tube.
When air is pumped out of a tube in which there is an electric spark discharge, several different stages, as shown in Fig. 3, are successively reached. At first the discharge in the tube is the ordinary disruptive spark. In the next stage it forms a quiet thread of light thru the tube. Then the thread of light becomes a broad pencil of light, and finally with more exhaustion the whole tube is filled with a soft luminous glow, which is technically known as the positive column. This is the point of highest conductivity of the tube, and may be reached with an ordinary air pump. With further exhaustion, by means of a special air pump, the resistance increases, the cold light disappears, and the point is reached" where X-ray phenomena and fluorescence appear.. The color of the light in the tube depends on the gas used in the tube and is undoubtedly the product of the ions. Just before the war a Frenchman, Claude, devised a means of producing in quantities the rare gas, neon, which gives an intense yellow light in a vacuum tube. The carbon dioxid which Moore uses gives a white light. Other gases, possibly rare ones, may be found which are better still, and helium has been suggested as a standard by some. However, up until the time the U. S. Government discovered the way of producing it in immense quantities for balloon work, during the war, it was too rare to be practical. The more the conditions in such a light can be improved, and the more nearly the light is a pure ionization or cold light, the higher the luminous efficiency will be. An interesting table is given, showing the progress which has been made up to the present, including the vacuum tube.
Luminous Efficiency of Various Illuminants
| Candle | 2 per cent |
| Gas mantle | 5 per cent |
| Carbon lamp | 6 per cent |
| Flaming arc | 15 per cent |
| Vacuum tube | 40 per cent |