TCBA Volume 3 - Issue 1
Page 12 of 18
Experiments with High Potentials at High Frequency
(part 6)
Even at high frequencies, experimenting with high potentials is not without danger. Readers are urged to review the suggested precautions listed in Part 1 of Volume 1 #2, page 14.
Fun with Pyrex Flasks (or glass jars)
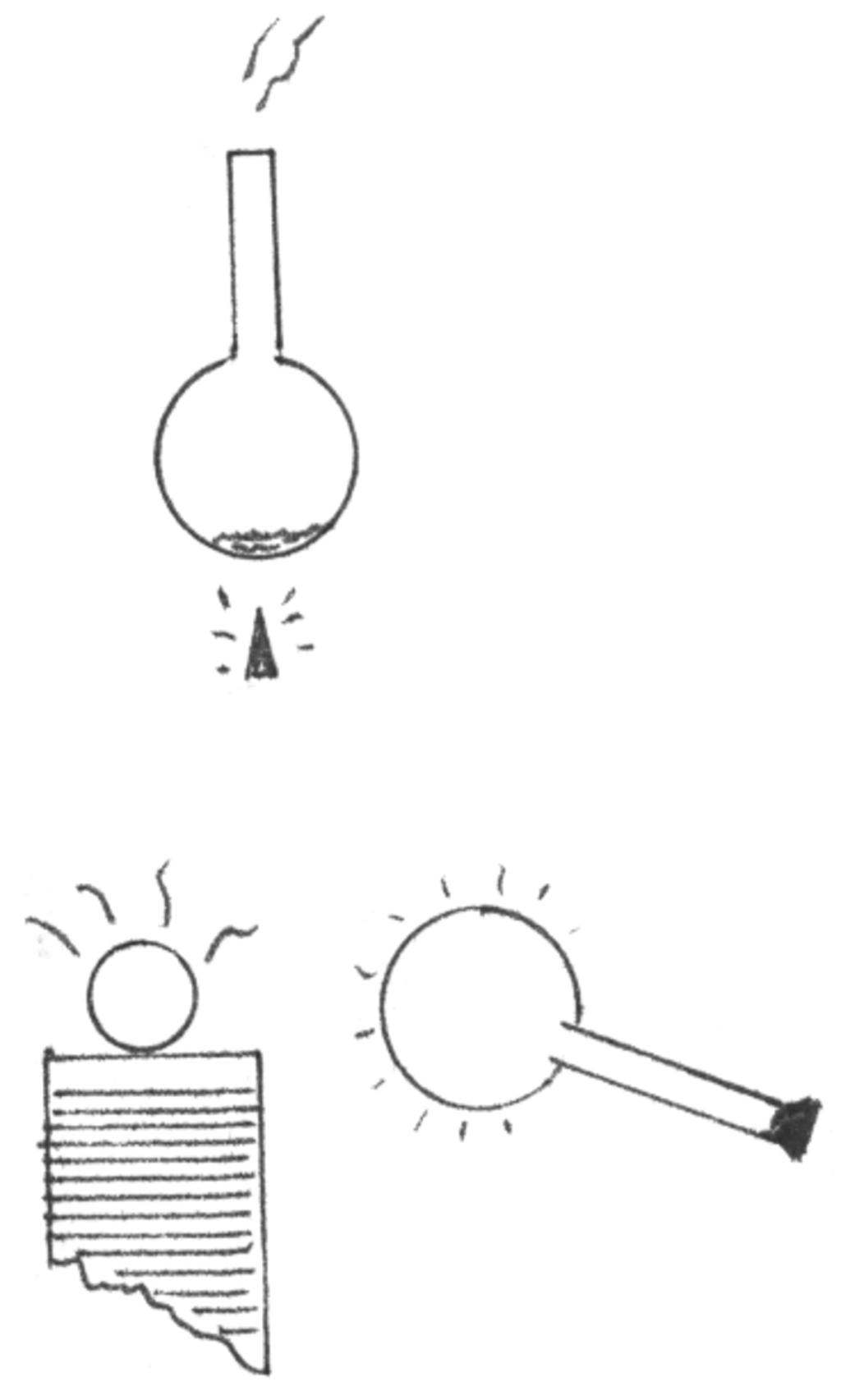
13. Place enough water into a flask to a depth of about 1/4". Heat to a boiling point and allow the water to evaporate.
Remove the flask and seal with a rubber stopper and allow to cool. Energize a Tesla coil and bring the flask in the vicinity of the discharges. The flask will glow.
This experiment will work best in the dark as the glow from the flask will be weak. Since the space within the flask is at a reduced pressure, there is room for the particles to move enough to reach rapid speeds. The glow comes from the collision of particles.
The flask will produce different hues depending upon the amount of reduced pressure. If you have a vacuum pump, merely remove the air within by suction.
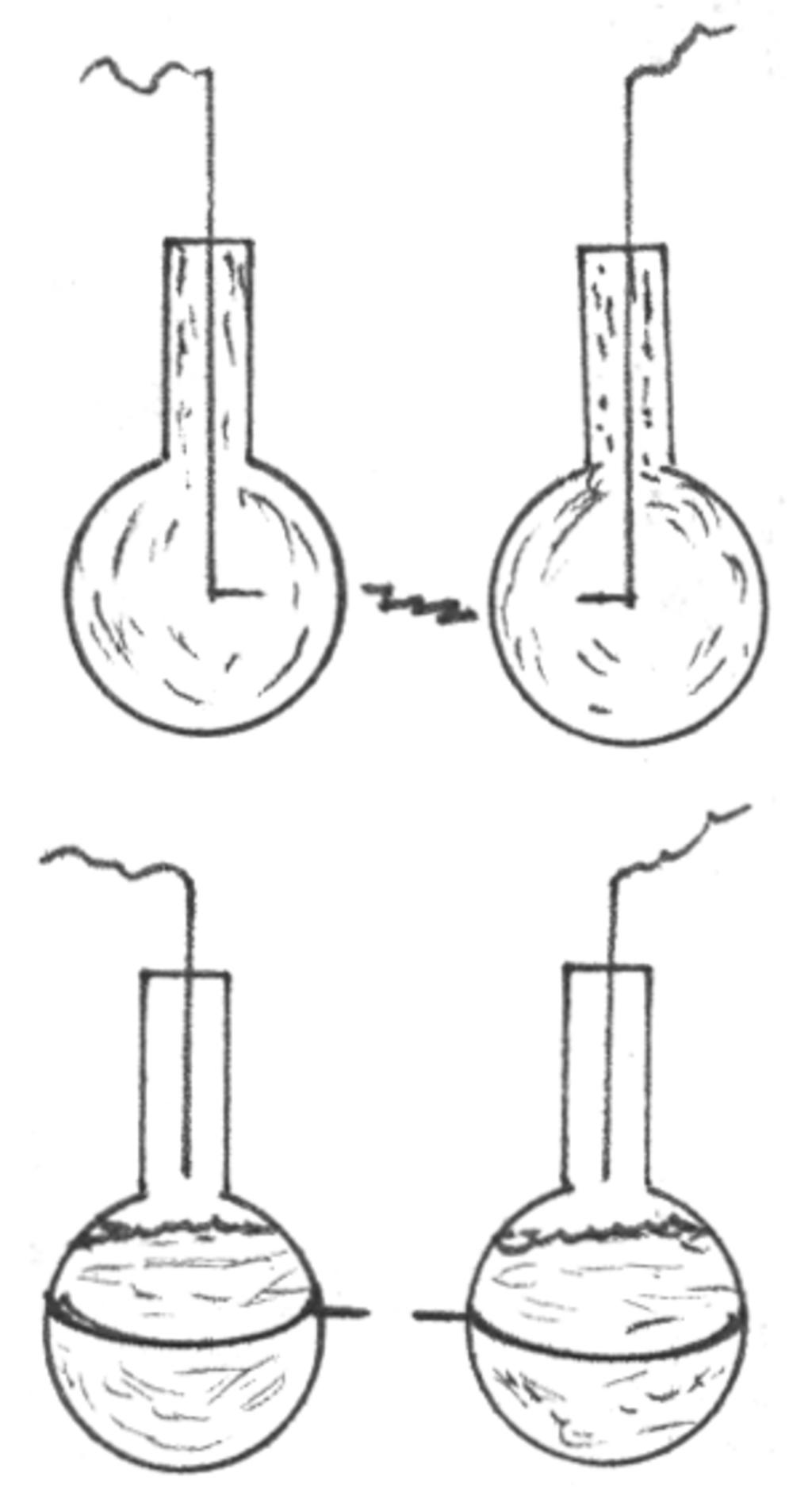
14. Fill two jars or flasks with water and insert a connecting wire in each as shown in the diagram. Connect the containers to the two Tesla coil terminals (or one terminal and the ground).
A spark will jump through the water and glass when the coil is functioning.
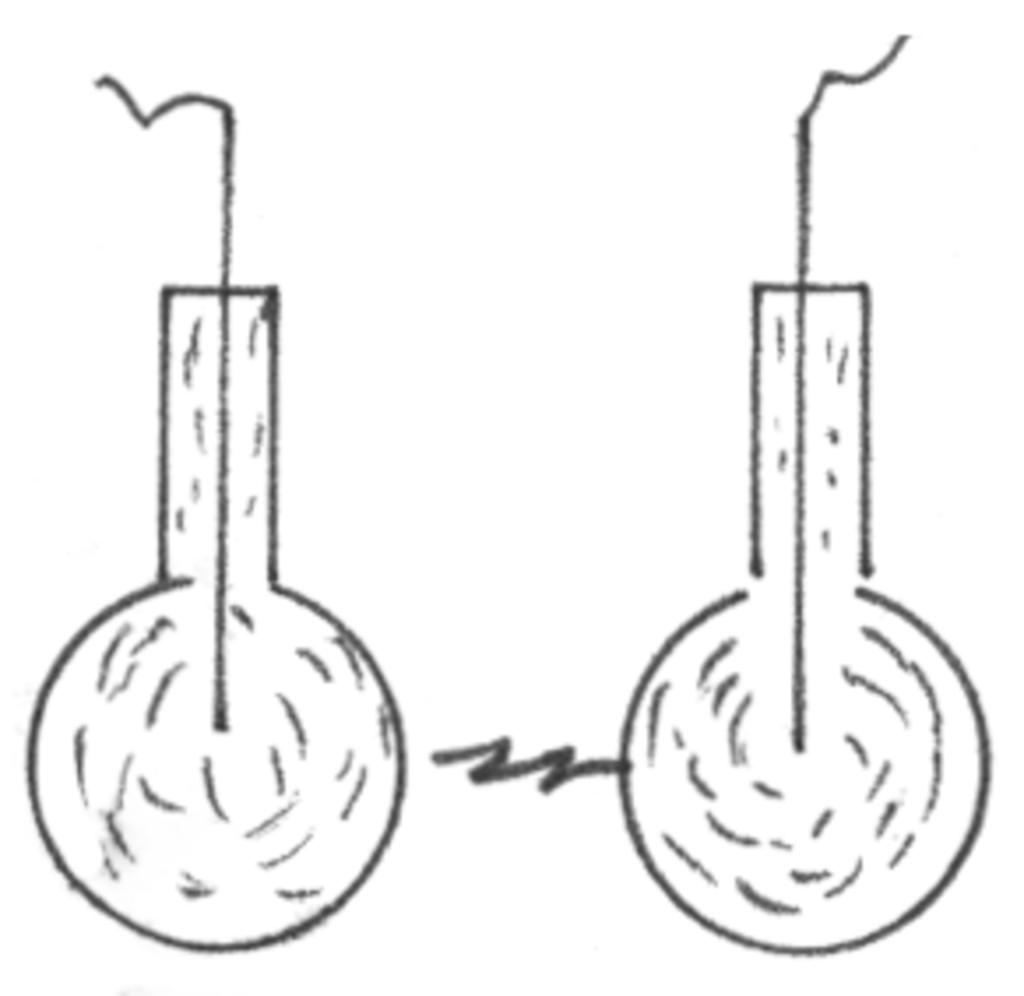
Rearrange the connections as shown in the diagram at left. A spark will jump between the gap even though the wires are only wrapped around the jars and unconnected to the Tesla coil.
15. An interesting result will occur if the solutions in the flasks fluoresce. One such example is to add a few drops of a coal tar based chemical (such as fluorescein) to one flask that has been filled with water. To the other flask of water, add a few drops of bi-sulfate of quinine.
Place the containers a few inches apart and connect to a Tesla coil. When the coil is energized, the ultra violet rays from the sparking that occurs between the flasks or jars will cause fluorescence of the solutions. The colors will be pale blue from the quinine and greenish in the coal tar.